Scaling IT compliance in pharma and life sciences is complex, regulated, and time-critical. Traditional processes couldn’t keep up with evolving standards, manual errors, and the pressure to deliver fast, error-free audits. To overcome this, SagaxTeam partnered with GoML to build a Generative AI-powered system for automated audits using a pharma AI compliance agent.
The problem: manual audits, regulatory risk, and lack of scalability
As a leading provider of managed solutions for pharma and biotech firms, SagaxTeam needs to scale IT system audits without compromising accuracy or compliance. But their manual approach was slow, error-prone, and unfit for the demands of global regulations like FDA 21 CFR Part 11, GxP, and GDPR. Auditors had to sift through structured and unstructured data, interpret compliance requirements, and document findings manually. This approach was not only time-consuming but also introduced critical risk factors, including inconsistent assessments and missed compliance gaps.
SagaxTeam needed an intelligent, adaptive pharma AI compliance agent solution to drive speed, precision, and consistency across every pharma and biotech IT audit.
The solution: a Gen AI pharma compliance agent
GoML worked with SagaxTeam to design and deploy a domain-specific AI assistant for IT audits and compliance, tailored for the Life Sciences industry. This pharma AI compliance agentic system combines large language models, automated documentation, and real-time risk assessments, purpose-built for an AI-powered IT audit for pharma and biotech.
Generative AI for data interpretation and context building
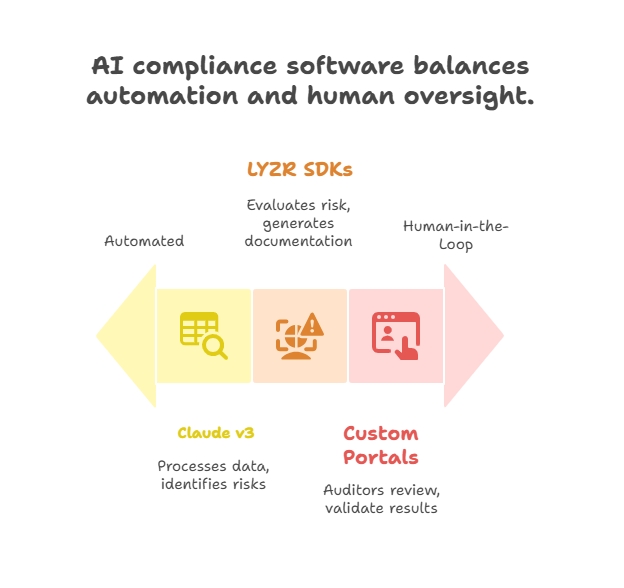
Claude v3, hosted on AWS EC2, processes structured and unstructured IT system data to identify key compliance markers, assess system criticality, and flag potential risks, building contextual intelligence across audits through the pharma AI compliance agent.
Dynamic risk assessment and documentation generation
Using LYZR SDKs integrated with retrieval-augmented generation (RAG) pipelines, the system dynamically evaluates risk levels and auto-generates tailored compliance documentation aligned with the latest standards, enhancing the adaptability of the pharma AI compliance agent.
Reviewer and user portals for human-in-the-loop validation
Custom portals allow auditors to upload IT system details, review AI-generated reports, and validate results, ensuring transparency and expert oversight in every IT audit.
The impact: audits at 10x speed, zero-error compliance at scale
- 70% of the audit preparation and review process is now fully automated, saving valuable analyst time through the pharma AI compliance agent
- 50% reduction in documentation errors, resulting in more consistent and compliant audit reports
- 80% faster audit completion times, enabling SagaxTeam to scale with confidence across global clients
Lessons for pharma, biotech, and regulated IT environments
Common pitfalls to avoid
- Relying solely on human effort to process complex compliance data
- Using static templates that don’t adapt to evolving global regulations
- Lacking a feedback loop between system data and documentation outputs
Tips for compliance and engineering teams
- Start with domain-specific LLMs for contextual risk interpretation
- Integrate RAG pipelines for real-time documentation generation
- Use human-in-the-loop portals to balance automation with expert validation
Ready to modernize your IT audits with AI agents?
Let GoML help you design and deploy an AI compliance agent for IT audits in pharma and biotech, purpose-built for speed, accuracy, and regulatory confidence.
Reach out to create your own Gen AI-powered audit and compliance assistant.

.png)
.png)


